38 fda approved health claims on food labels
Select the correct suggestion that support the key recommendations in ... All of the following are FDA approved health claims, except for: Vitamin D may reduce the risk of depression. ... Drinking fluoridated water may reduce the risk of dental caries" is an FDA approved health claim that can be used on food labels. Added 5/21/2021 3:13:11 AM. Sen. Johnson Demands DoD, FDA, & CDC Address COVID Label Fraud ... senator ron johnson has given the top brass of the u.s. department of defense (dod), the food and drug administration (fda), and the centers for disease control and prevention (cdc) until september 1, 2022, to provide detailed information on potential fraud surrounding the labeling of the pfizer covid-19 jabs being forced upon our nation's …
How to Read Food Labels: Your Complete Consumer Guide In the United States, all front-of-package labels are voluntary, which allows food manufacturers to highlight or ignore nutrition information to help promote or preserve sales. While food labels can throw around generic terms like "natural" and "pure" with abandon, they're severely restricted in the health claims they can make.

Fda approved health claims on food labels
Environmental claims on feed labels: FDA to host virtual event to gain ... Paul Davis, director of quality, animal food safety, and education, AFIA, presenting at the US Department of Agriculture (USDA) Agricultural Outlook Forum in February, said part of the problem is that novel feed ingredients in the US cannot include environmental claims on their labels due to the FDA's very narrow interpretation for what is ... Honduras | Food Safety and Inspection Service The USDA, Animal and Plant Health Inspection Service (APHIS) restricts certain animal products from entering the United States because of animal disease conditions in the country of origin. Applicable APHIS animal disease requirements that may have an impact on Honduras's eligibility to export product to the United States are listed below: Resource Grains - USDA The product includes one of the following FDA-approved whole grain health claims on its packaging and any refined grains in the product are enriched: "Diets rich in whole grain foods and other plant foods and low in total fat, saturated fat, and cholesterol may reduce the risk of heart disease and some cancers."
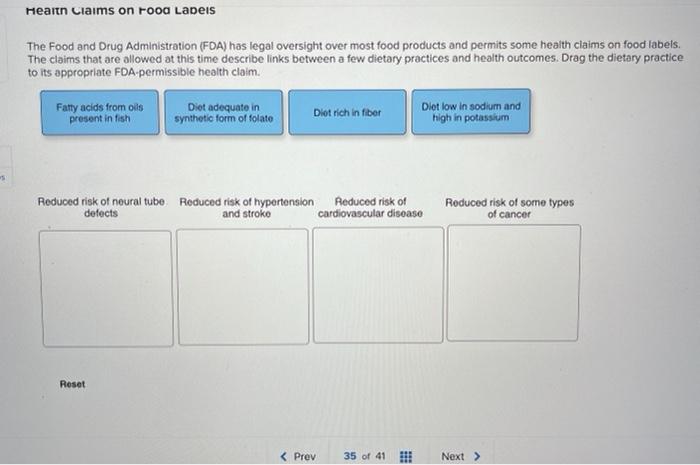
Fda approved health claims on food labels. Nutrition labelling | Food Standards Agency This scheme is recognised as an important tool in supporting consumers to better understand the nutrient content of their food and drink. The FSA in partnership with Department of Health and Social... Economic Impact Analyses of FDA Regulations | FDA - U.S. Food and Drug ... Food Labeling: Health Claims; Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease (Interim Final Rule) - December 19, 2016 General Hospital and Personal Use Devices: Renaming of Pediatric Hospital Bed Classification and Designation of Special Controls for Pediatric Medical Crib; Classification of Medical Bassinet (Final ... Resource Appendix E - USDA Is the food product labeled with an FDA-approved whole grain health claim? If a food product is labeled with an FDA-approved health claim, the product label will include one of the following on its packaging: "Diets rich in whole grain foods and other plant foods and low in total fat, saturated fat, and cholesterol may reduce the risk of ... › nutritionsource › food-labelUnderstanding Food Labels | The Nutrition Source | Harvard T ... The FDA has approved 12 health claims on food labels such as the relationship between calcium and osteoporosis; sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and folic acid and neural tube defects. However, just because a food contains a specific nutrient that is associated with a decreased risk of disease ...
How FDA Failures Contributed to the Opioid Crisis The Food, Drug, and Cosmetic Act requires “adequate and well-controlled studies” before products can be approved and promoted as safe and effective. 13 The FDA generally requires at least 2 randomized controlled trials demonstrating clear efficacy for a proposed indication. 24 Yet it approved extended release oxycodone based on only one adequate and well-controlled … Nearly 60% Of CBD Products Are Mislabeled, A New Study Finds, With Some ... Swift actions and reform by regulatory agencies (e.g., the Food and Drug Administration, FDA) have the potential to ensure label accuracy and alleviate concerns of vulnerable consumers who rely on ... FDA Approves RECOVER IV Randomized Controlled Trial with Exception from ... DANVERS, Mass., September 16, 2022--(BUSINESS WIRE)--Abiomed(Nasdaq: ABMD) announces two approvals from the U.S. Food and Drug Administration (FDA) related to clinical research of Impella heart... CFR - Code of Federal Regulations Title 21 - Food and Drug … 29.03.2022 · (iii) Claims described in paragraph (b)(5) of this section may not be made on the label or labeling of food if the reference food meets the definition for "low calorie." (c) Sugar content claims - (1) Use of terms such as "sugar free," "free of sugar," "no sugar," "zero sugar," "without sugar," "sugarless," "trivial source of sugar," "negligible source of sugar," or "dietarily …
Glyphosate | US EPA As part of the human health risk assessment, the Agency evaluated all populations, including infants, children and women of child-bearing age, and found no risks of concern from ingesting food with glyphosate residues. EPA also found no risks of concern for children entering or playing on residential areas treated with glyphosate. Is It Really 'FDA Approved'? - U.S. Food and Drug Administration 10.05.2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ... Is Pfizer's FDA-approved COMIRNATY Vaccine Available in the US? An FDA spokesperson said, "There are two formulations of the Pfizer-BioNTech covid-19 vaccine authorized for emergency use for individuals 12 years of age and older, and these same formulations are also approved under the COMIRNATY license for individuals 16 years of age and older." › it-really-fda-approvedIs It Really 'FDA Approved'? | FDA - U.S. Food and Drug ... May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ...
Nutrition Facts Label - IFT.org - Institute of Food Technologists The first Nutrition Facts Label regulations were published in 1993 and launched in 1994. More than two decades later, in 2016, the U.S. Food and Drug Administration (FDA) released new requirements for the Label to provide recent and accurate nutrition information about foods based on updated scientific data and more recent consumer behavior trends.
China | Food Safety and Inspection Service Food Safety. Recalls & Public Health Alerts. Report a Problem with Food. Additional Recalls; ... Labeling. Basics of Labeling; Claims Guidance; ... and seal and container numbers before the application can be approved. Approved export certificates (FSIS Forms 9060-5/9060-5S) and any continuation pages (FSIS Forms 9060-5A/9060-5B) must be signed ...
FDA Safety Changes: Statin Label Revised With New Warnings - Medscape Upon completion of this activity, participants will be able to: Describe FDA-approved safety labeling changes for statins regarding the monitoring of liver enzymes. Describe the new adverse events information added to the FDA-approved safety labeling changes for statins. Describe the updated changes to the FDA-approved safety label for lovastatin.
What safeguards are in place to protect supplement consumers, and are ... When it comes to advertising, the FDA is responsible for oversight of claims made on labels and packaging, but the Federal Trade Commision (FTC) oversees online and print advertising, including ...
What Is a Whole Grain? - Academy of Nutrition and Dietetics Offering whole grains with at least 8 grams or more per serving. Buying products that include the FDA-approved whole-grain health claim on its packaging. Making sure that product ingredient lists state whole grains first. Try swapping out refined grains and white breads and pastas for whole-grain varieties.
› food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Food Legislation / Guidelines - CFS The basic food law in Hong Kong is laid down in Part V of the Public Health and Municipal Services Ordinance (Cap. 132). The main provisions cover general protection for food purchasers, offences in connection with sale of unfit food and adulterated food, composition and labelling of food, food hygiene, seizure and destruction of unfit food ...
› scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · (4) For dietary supplements, claims regarding calories may not be made on products that meet the criteria in § 101.60(b)(1) or (b)(2) for "calorie free" or "low calorie" claims except when an equivalent amount of a similar dietary supplement (e.g., another protein supplement) that the labeled food resembles and for which it substitutes ...
COVID-19 Vaccines | FDA - U.S. Food and Drug Administration COVID-19 Vaccines Authorized for Emergency Use or FDA-Approved Pfizer-BioNTech COVID-19 Vaccines Moderna COVID-19 Vaccines Janssen COVID-19 Vaccine Novavax COVID-19 Vaccine, Adjuvanted Fact sheets...
Emergency Use Authorization of Medical Products this guidance explains fda's general recommendations and procedures applicable to the authorization of the emergency use of certain medical products under sections 564, 564a, and 564b of the...
Text of H.R. 1599 (114th): Safe and Accurate Food Labeling Act of 2015 ... Text of H.R. 1599 (114th): Safe and Accurate Food Labeling … as of Jul 24, 2015 (Referred to Senate Committee version). H.R. 1599 (114th): Safe and Accurate Food Labeling Act of 2015
Label Claims for Conventional Foods and Dietary Supplements 07.03.2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
CFSAN Constituent Updates | FDA - U.S. Food and Drug Administration 1/10/2022 - FDA Announces Qualified Health Claim for Magnesium and Reduced Risk of High Blood Pressure; ... 10/6/2020 - FDA Seeks Input on Labeling of Food Made with Cultured Seafood Cells;
Can Eating Oats Reduce Cholesterol? - joinzoe.com Today, the links between beta-glucan and cholesterol levels are well enough established that several official bodies have approved related health claims. For instance, the United States Food and Drug Administration (FDA) allows food manufacturers to advertise that their oat products are heart healthy.
Minimum Risk Pesticides Exempted from FIFRA Registration Conditions to Qualify as a Minimum Risk Pesticide Product. Minimum risk conditions. Active ingredients. Inert ingredients. Listing ingredients on the label. Public health claims. Company name and contact information. False or misleading labeling statements.
journalofethics.ama-assn.org › article › how-fdaHow FDA Failures Contributed to the Opioid Crisis | Journal ... The Food, Drug, and Cosmetic Act requires drug manufacturers to demonstrate that their products are both safe and effective before they are marketed. 13 The benefits of a drug must outweigh potential risks for specific indications listed on an FDA-approved label. 13 Although prescribing medication for unapproved uses is common and sometimes ...
Food supplements | Food Standards Agency the business name and address, which can be placed either on the product label or product packaging. This must be either: (a) the name of the business whose name the food is marketed under; or (b)...
September 2022 decisions expected from the FDA - Prime Therapeutics Our clinical and trade relations teams keep a keen eye on drugs likely to be approved by the U.S. Food and Drug Administration (FDA). Drug pipeline for September 2022: 9/9/2022: Rolontis ® (eflapegrastim) The FDA is reviewing Spectrum Pharmaceuticals and Hanmi Pharmaceutical's Rolontis as a long-acting granulocyte colony-stimulating factor ...
National Drug Code Directory - Food and Drug Administration U.S Department of Health and Human Services Public Health Service Food and Drug Administration ... FOIA; HHS.gov; USA.gov; Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds. FDA Homepage. Contact Number 1-888-INFO-FDA (1-888-463-6332)
Resource Grains - USDA The product includes one of the following FDA-approved whole grain health claims on its packaging and any refined grains in the product are enriched: "Diets rich in whole grain foods and other plant foods and low in total fat, saturated fat, and cholesterol may reduce the risk of heart disease and some cancers."
Honduras | Food Safety and Inspection Service The USDA, Animal and Plant Health Inspection Service (APHIS) restricts certain animal products from entering the United States because of animal disease conditions in the country of origin. Applicable APHIS animal disease requirements that may have an impact on Honduras's eligibility to export product to the United States are listed below:
Environmental claims on feed labels: FDA to host virtual event to gain ... Paul Davis, director of quality, animal food safety, and education, AFIA, presenting at the US Department of Agriculture (USDA) Agricultural Outlook Forum in February, said part of the problem is that novel feed ingredients in the US cannot include environmental claims on their labels due to the FDA's very narrow interpretation for what is ...






























Post a Comment for "38 fda approved health claims on food labels"